Sn2 Sn1 E2 and E1 Made Easy
SN1/SN2/E1/E2 Decision
By James Ashenhurst
Wrapup: The Quick N' Dirty Guide To SN1/SN2/E1/E2
Last updated: February 23rd, 2021 |
The Quick N' Dirty Guide To SN1/SN2/E1/E2 : Putting It All Together
The previous several posts dealt with an approach to solving substitution and elimination problems that can only be described as a Quick N' Dirty Guide to SN1/SN2/E1/E2. The basic premise is this: given 15-20 minutes to describe the basic principles by which one could figure out if a given reaction goes down one of these pathways, these are, in my opinion, the key factors to consider.
Quick N' Dirty rules, by their nature, do not cover exceptions. To learn about some of the exceptions, I advise you to go back and read the individual posts [One Two Three Four].
Even further back, I urge you to understand the key concepts behind each reaction, such as nucleophilicity, leaving group ability, carbocation stability, and the mechanism of each of these reactions. [SN1] [SN2] [E1] [E2]
Finally, I will preface this by saying that the best way to learn and understand how these reactions work is to do a lot of practice problems and pay particular attention to situations where you get the wrong answer – they are instructive.
Here goes:
Question 1: Is the carbon containing the leaving group methyl (only one carbon), primary, secondary, or tertiary?
- Quick N' Dirty Rule #1: If primary, the reaction will almost certainly be SN2 [prominent, commonly encountered exceptions: 1) a bulky base such as tBuOK will tend to give elimination products [E2]; 2) primary carbons that can form relatively stable carbocations (i.e. allylic/benzylic) may proceed through the SN1/E1 pathway.] Also – methyl carbons always proceed through SN2.
- Quick N' Dirty Rule #2: If tertiary, the reaction cannot be SN2. [Because tertiary alkyl halides are too hindered for the SN2. Depending on the type of nucleophile/base, it will either proceed with concerted elimination [E2] or through carbocation formation [SN1/E1]
Question 2: Does the nucleophile/base bear a negative charge?
- Quick N' Dirty Rule #3: Charged nucleophiles/bases will favor SN2/E2 pathways [i.e. rule out SN1/E1]. [So, for example, if SN2 has already been ruled out [e.g. for a tertiary carbon, according to Question 1] then the reaction will therefore be E2. This is the case for tertiary alkyl halides in the presence of strong bases such as NaOEt, etc. The strength of the [charged] nucleophile/base can be important! An important special case is to be aware of charged species that are weak bases [such as Cl, N3, –CN, etc.] these will favor SN2 reactions over E2 reactions].
- Quick N' Dirty Rule #4: If a charged species is not present, the reaction is likely to be SN1/E1. [so if the only reagent is, say, H2O or CH3OH you are likely looking at carbocation formation resulting in an SN1/E1 reaction.]
Question 3: Is the solvent polar protic or polar aprotic?
- Quick N' Dirty Rule #5: All else being equal, polar aprotic solvents favor substitution [SN2] over elimination [E2]. Polar protic solvents favor elimination [E2] over substitution [SN2].[Note that this rule is generally only important in the case of trying to distinguish SN2 and E2 with a secondary alkyl halide and a charged nucleophile/base. This is not meant to distinguish SN1/E1 since these reactions tend to occur in polar protic solvents, which stabilize the resulting carbocation better than polar aprotic solvents.]
Question 4: Is heat being applied to the reaction?
- Quick N' Dirty Rule #6: Heat favors elimination reactions. [This only becomes an important rule to apply when carbocation formation is indicated and we are trying to decide whether SN1 or E1 will dominate. At low temperatures SN1 products tend to dominate over E1 products; at higher temperatures, E1 products become more prominent.]
Writing this post makes me feel like a nun giving out condoms. I realize there will be many who are reading this an hour before their exam and are completely clueless on this subject. All I have to say is, God help you. And do more fricking practice problems so you don't put yourself in this situation next time.
Struggling with SN1/SN2/E1/E2? Our Org 1 Summary Sheets (PDF) contain a full-page flowchart on deciding SN1/SN2/E1/E2, as well as two more pages summarizing substitution and elimination reactions, in addition to many other Org 1 topics.
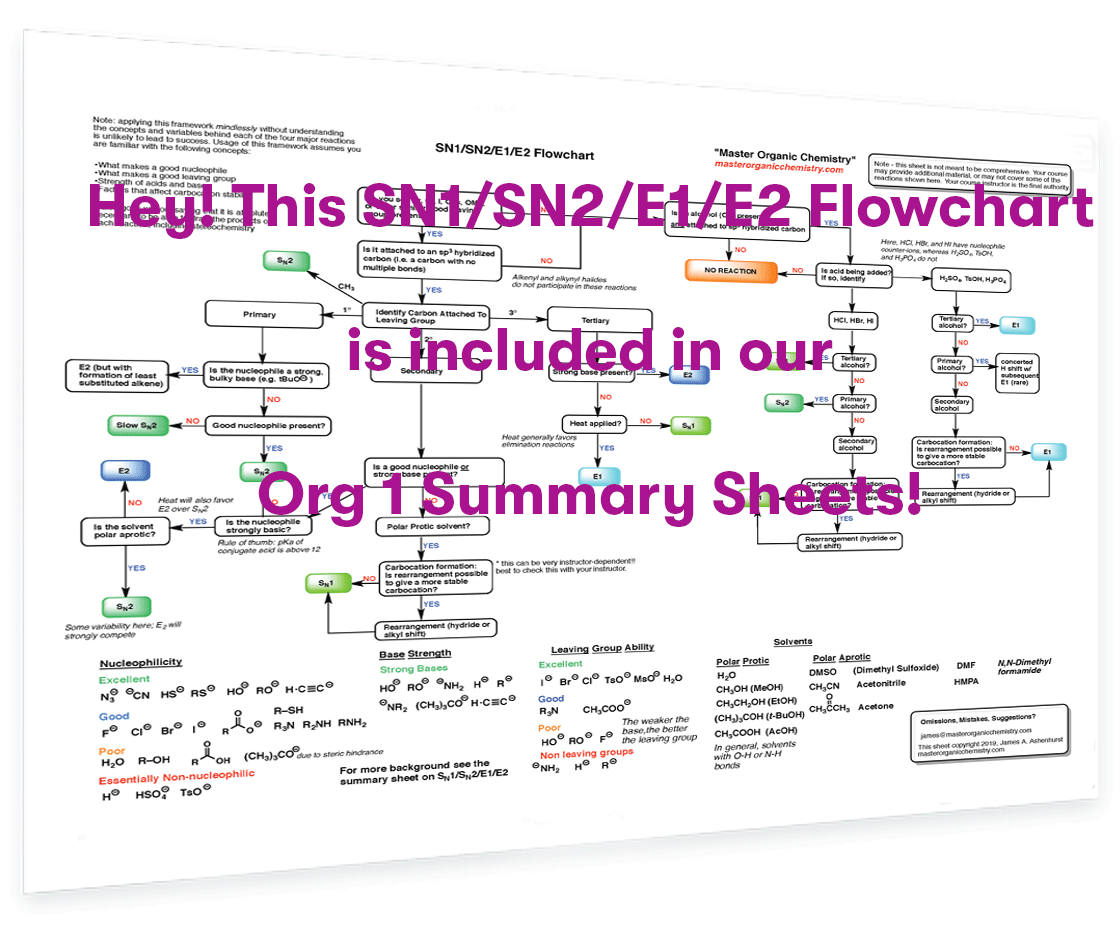
Check them out now!
frisbydoorguichat.blogspot.com
Source: https://www.masterorganicchemistry.com/2013/01/18/wrapup-the-quick-n-dirty-guide-to-sn1sn2e1e2/
0 Response to "Sn2 Sn1 E2 and E1 Made Easy"
Post a Comment